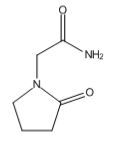
Piracetam was the first racetam to be synthesized by Dr Giurega in 1964 and was which the term “nootropic” was born
Piracetam was found to mildly enhance verbal memory after 2 weeks use at 3.6g / day in healthy adults and had a modest increase (8.6%) in verbal learning during a 21 day trial of 1.6g /day in student volunteers.
Piracetam is generally beneficial for cognitive decline associated with age.
Piracetam taken orally has nearly 100% plasma bioavailability, meaning virtually all of it makes it to the bloodstream unchanged. It is rapidly absorbed by the body, and reaches peak plasma concentration within 30 minutes when taken by fasting subjects. Prescription dosages for cognitive disorders and vertigo are typically around 2.4-4.8 g daily, p.o. (per os, “by mouth”). Cortical myoclonus treatments are around 7.2-24.0 g daily, p.o. For a 2 g p.o. dose to humans, the CNS half-life is 7.7 hours, and the plasma half-life is 5 hours. Without re-administration, piracetam leaves the body entirely in 30 hours.
Dosing Piracetam
In a study looking at dosage-related efficacy, researchers administered subjects affected by age-associated memory impairment (AAMI) with 3 different levels of dosing: placebo, 2.4 g/day, and 4.8 g/day (55+ years old; placebo wash-out period of 10 days; 3 parallel groups of 45 participants completed). The researchers found that 1) therapy was most effective in those with the worst baseline performance, and 2) of the three dosing levels, 4.8 g/day was the most effective.[5]
Piracetam Background
While the compound has existed since the mid 1960s as a treatment for motion sickness, the 1971 discovery of piracetam’s neuroprotective effects in mouse models effectively kickstarted the nootropic movement. Piracetam falls into a class of similarly-structured nootropics, racetams. All can pass through the blood-brain barrier to a certain degree. While members of this class are structurally similar, the small differences are enough to yield a range of different chemical properties.[3]
Piracetam’s Mode of Action
Though piracetam (nootropil) has held the longest market awareness of nootropics, its mode of action is still unclear. A few hypotheses:
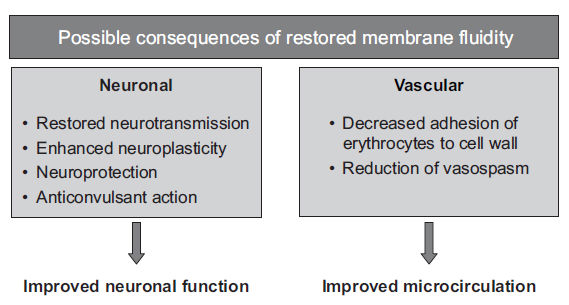
- Restoration of membrane fluidity of compromised neuronal cells, higher membrane fluidity ultimately allows signaling molecules to more easily cross the lipid bilayer membrane, which is an integral step in cell signalling (more relevant to our interests, neurotransmission); piracetam, in concert with calcium ions (an ion important to cell signalling), appears to effect physical changes in membranes.
- Steroid-sensitive protein synthesis-modulation where protein-synthesis is a part of memory-storage/consolidation; adrenolectomized rats didn’t demonstrate the memory-enhancing effects when given several racetams at different dosage levels, but they could still learn; steroids may play a part in transporting the nootropics through the membrane. Also, when steroid receptor activity (adrenal function) is suppressed by drugs, the different racetams also don’t ‘work’. Amnestic effects seem to be dependent on the adrenal medulla.
- Interaction with glutamate neurotransmission – potentially binding with glutamate receptors; possibly recruitment of AMPA receptors normally not part of the synaptic transmission.
- Interaction with acetylcholine neurotransmission – increase in cortical muscarinic receptor density (aged rats)
Piracetam Benefits
Preclinical studies (mainly rat models) have shown piracetam to do the following:
- Enhance subject performance in a standard, passive-avoidance test (learning and memory)
- Protect against shock/hypoxia-induced amnesia (neuroprotection)
- Reduce alcohol and alcohol-withdrawal related neuronal loss, when neural circuitry is recoverable (neuroplasticity).
The drug has applications in treating cognitive disorders, vertigo, cortical myoclonus (involuntary, spontaneous muscle movements), dyslexia, and sickle cell anemia.
The magnitude of piracetam’s cognitive-enhancement varies depending on the individual. Studies have shown more noticeable effects/improvements in aged vs. young animals.
Piracetam Toxicity
Regarding toxicity, in preclinical trials, there were no irreversible toxic effects in mice, rats, or dogs in single oral doses up to ~4.5 g/lb. Controlled human clinical trials have shown high dosage tolerability, with a typical, no side effect dose in the range of 2.4-4.8 g/day p.o., with “no side effects” being interpreted as a drop-out rate comparable with the control/placebo group. Doses up to 8.0 g/day given to subjects suffering from Alzheimer’s also yielded drop-out rates comparable to the placebo group.[6] While reproductive studies have shown no risk to fetuses in animal studies, no comparable trials have been conducted for pregnant or lactating women.
Until recently, piracetam’s recreational applications fell under the category of nutritional supplements; thus, there used to be fairly lax FDA regulation. After further scrutiny, the FDA served warning letters to piracetam manufacturers and distributors in the US notifying them of piracetam’s exclusion from the dietary supplement definition.
Not to be confused with Toxicity, but there have been some reports of adverse psychiatric responses to Piracetam.
Other information
On piracetam’s synergy with choline
A study was conducted in 1981 to explore the potential synergy in administering choline (a precursor molecule for neurotransmitter acetylcholine) jointly with piracetam for memory-enhancement in rats, using a rat species whose memory noticeably deteriorated with age. The memory loss that increases with age in this species is similar to mice, old world monkeys, new world monkeys, and humans, hence, the researchers’ choice of using this model system. Researchers took a sample of aged (20+ months) rats, and separated them into 4 treatment arms:
- Control (tap water)
- Piracetam (200 mg/kg per day)
- Choline (200 mg/kg per day)
- Piracetam/choline combination (100 mg/kg of each per day)
Fluid treatments were administered chronically for 1 week via drinking water, where the control was tap water.
The well-established passive-avoidance task consists of a two-chamber testing apparatus, with the two chambers separated by an initially shut guillotine door (figure below); one chamber is lit, and the other is not. The test subject is introduced into the light chamber, and allowed to explore for 3 seconds for orientation. The guillotine door is opened. After the rat enters the unlit chamber, the door is shut, and an electric shock is delivered to the dark chamber floor grids for 3 seconds. The rat is then removed from the apparatus and returned to its cage for 24 hours.
